box of electros We can note the configuration and orientation of the electrons visually using box notation. It’s usually easiest to use the Aufbau principle (or the periodic table!) to write down the electronic configuration and then translate it to box notation. To avoid this, cancel and sign in to YouTube on your computer. These videos from Kenney will help you install your curtain rods.
0 · outer electron box diagram
1 · orbital diagram cheat sheet
2 · orbital box diagram for carbon
3 · how to draw electron configuration
4 · electrons in boxes notation
5 · electron in box diagram
6 · box diagram electron configuration
7 · box and arrow diagram chemistry
Z-Wave Products. There are over 4401 interoperable Z-Wave products throughout the world, and over 94 million Z-Wave products have been sold since our beginnings in 2001. All of them .
outer electron box diagram
We can show the distribution of electrons by using box diagrams, where each box represents an orbital and the arrows within the boxes represent the electrons in that orbital. The direction of the arrow represents the spin of the .Boxes, or horizontal lines represent the orbitals, arrows represent the electrons, and if an orbital is full, the electrons must be of opposite spin–one arrow pointing up and the other one pointing down. The orbital box diagrams are listed for the .
orbital diagram cheat sheet
The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to .
We can note the configuration and orientation of the electrons visually using box notation. It’s usually easiest to use the Aufbau principle (or the periodic table!) to write down the electronic configuration and then translate it to box notation.
Access detailed info on all elements: atomic mass, electron configurations, charges, and more. View rotating Bohr models for all 118 elements. Get a free HD image of the Periodic Table. Note: For future use, .
Jun 14, 2015
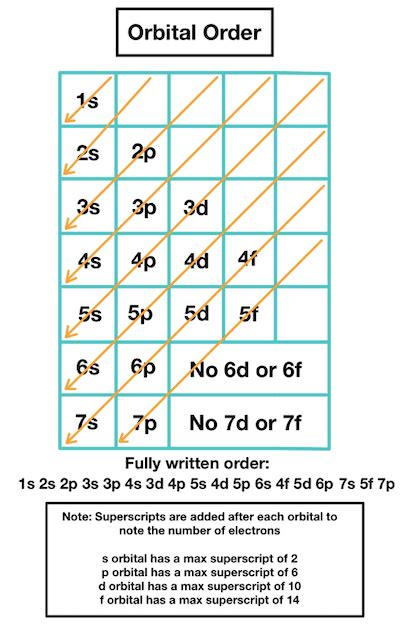
Electrons enter higher-energy subshells only after lower-energy subshells have been filled to capacity. Figure 6.26 illustrates the traditional way to remember the filling order for atomic orbitals. Each element has a unique atomic structure that is influenced by its electronic configuration, which is the distribution of electrons across different orbitals of an atom. This .
We can show the distribution of electrons by using box diagrams, where each box represents an orbital and the arrows within the boxes represent the electrons in that orbital. The direction of the arrow represents the spin of the electron.
orbital box diagram for carbon
Boxes, or horizontal lines represent the orbitals, arrows represent the electrons, and if an orbital is full, the electrons must be of opposite spin–one arrow pointing up and the other one pointing down. The orbital box diagrams are listed for the first 20 elements in the figure below.Give the electron configuration for an atom using Bohr’s model, box orbital diagrams, and quantum mechanical notation. The electron configuration describes the position of electrons of an atom or a molecule in atomic or molecular orbitals. It is a type of code that shows the number of electrons in each atom’s energy level and how they are arranged within each energy level.The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to .

We can note the configuration and orientation of the electrons visually using box notation. It’s usually easiest to use the Aufbau principle (or the periodic table!) to write down the electronic configuration and then translate it to box notation. Access detailed info on all elements: atomic mass, electron configurations, charges, and more. View rotating Bohr models for all 118 elements. Get a free HD image of the Periodic Table. Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com” The electron configuration states where electrons are likely to be in an atom. If you don’t have a chart, you can still find the electron configuration. Use the element blocks of the periodic table to find the highest electron orbital.
Electrons enter higher-energy subshells only after lower-energy subshells have been filled to capacity. Figure 6.26 illustrates the traditional way to remember the filling order for atomic orbitals.
Each element has a unique atomic structure that is influenced by its electronic configuration, which is the distribution of electrons across different orbitals of an atom. This article provides you with an electronic configuration chart for all these elements.
We can show the distribution of electrons by using box diagrams, where each box represents an orbital and the arrows within the boxes represent the electrons in that orbital. The direction of the arrow represents the spin of the electron.Boxes, or horizontal lines represent the orbitals, arrows represent the electrons, and if an orbital is full, the electrons must be of opposite spin–one arrow pointing up and the other one pointing down. The orbital box diagrams are listed for the first 20 elements in the figure below.Give the electron configuration for an atom using Bohr’s model, box orbital diagrams, and quantum mechanical notation. The electron configuration describes the position of electrons of an atom or a molecule in atomic or molecular orbitals. It is a type of code that shows the number of electrons in each atom’s energy level and how they are arranged within each energy level.
The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to .We can note the configuration and orientation of the electrons visually using box notation. It’s usually easiest to use the Aufbau principle (or the periodic table!) to write down the electronic configuration and then translate it to box notation.
Access detailed info on all elements: atomic mass, electron configurations, charges, and more. View rotating Bohr models for all 118 elements. Get a free HD image of the Periodic Table. Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com” The electron configuration states where electrons are likely to be in an atom. If you don’t have a chart, you can still find the electron configuration. Use the element blocks of the periodic table to find the highest electron orbital.Electrons enter higher-energy subshells only after lower-energy subshells have been filled to capacity. Figure 6.26 illustrates the traditional way to remember the filling order for atomic orbitals.
how to install metal roofing sheets
how to draw electron configuration
electrons in boxes notation
electron in box diagram
At APX York Sheet Metal, we’re proud of where we’ve been and excited about where we are going. We’ve been in business 70 years because we take care of our customers and deliver on our commitments — let us start delivering for you. All .
box of electros|box and arrow diagram chemistry